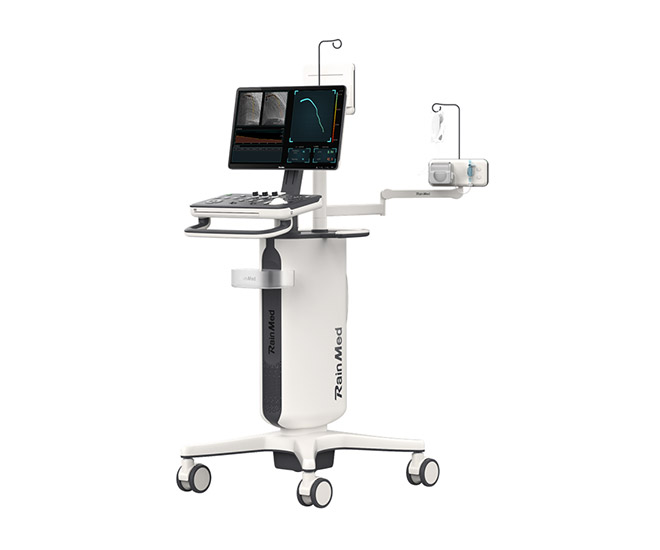
FlashAngio caIMR System
FlashAngio caIMR System (hereinafter referred to as caIMR) is a non-invasive microcirculatory lesion diagnosis and treatment system. The system has the characteristics of safety, efficiency, precision, simplicity, innovation, etc. Currently, caIMR has been classified as a Class III medical device by the Food and Drug Administration (FDA) and has officially started the clinical study. It is expected to be the first non-invasive coronary microcirculatory function measurement system in the world to obtain market access. (Medical device registration certificate has not yet been obtained).
Studies have confirmed that the combination of FFR and IMR can provide a complete functional evaluation of blood vessels from epicardial coronary arteries to intramyocardial microcirculation. caFFR and caIMR are currently used individually for the precise diagnosis of coronary artery disease and are subsequently expected to be central and key modules of our future panvascular interventional robots.
CONTACT US
0512-62622215
Address: Building 31, Northeast District, Nano City, No. 99 Jinji Lake Avenue, Suzhou industrial park
E-mail: market@rainmed.com

WeChat public account

Recruitment QR Code
Page Copyright© 2021- Suzhou Rainmed Medical Technology Co., Ltd. 苏公网安备32059002003601号 (苏)-非经营性-2021-0149

